CE 7smart UV On-/Offline
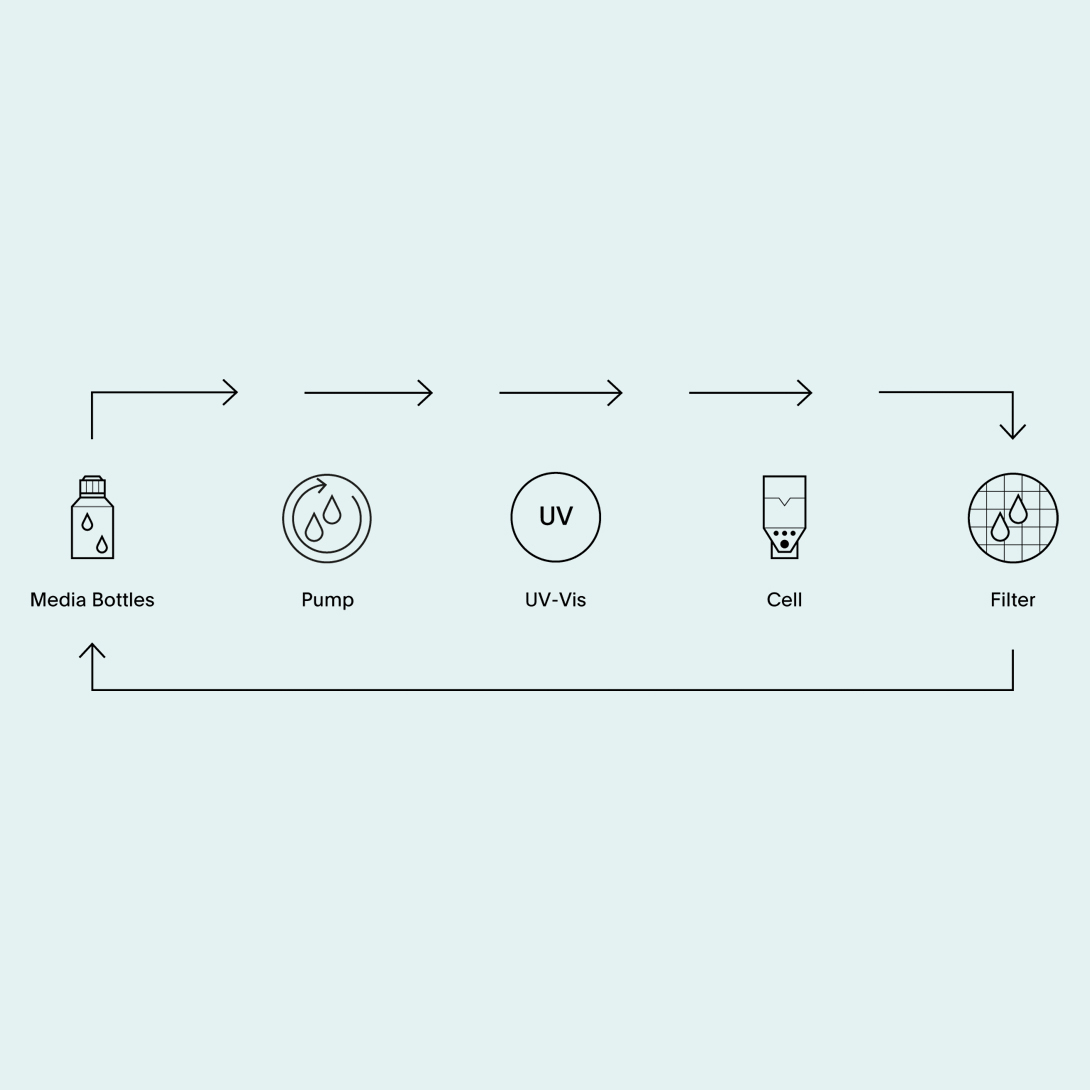
Flow-through cell dissolution testing system with integrated online UV-Vis analysis and offline sample collection.

- COLLECT OR ANALYZE – Delivers real time results and/or collect dissolution testing samples to alternate or compare your analytical finishes
- ADAPT – Test APIs, oral, topical and parenteral dosage forms reproducibly
- PROGRAM – Define methods and specifications in one validated platform, using SOTAX Dissolution software
- TEST – Allows a thorough method programming of every fraction and time point of your dissolution profile including flow rate and media changes
- RECORD – Allows complete traceability through user's right administration, audit trail, unlimited method programming and reporting
Highlights

Ready, set, disso
In addition, the combination of UV online measurement and offline sample collection is possible. If sample back up is needed or multi component products requiring a different analytical finish. The samples that have been measured in the photo spectrometer can subsequently be collected in the auto sampler. Combinations with different photo spectrometer types are available.

From API to novel dosage forms
Dissolution testing is a supra-indicator of parameters monitored during the manufacturing of a dosage form. The CE 7smart apparatus 4 widens the design of experiment being the only compendial dissolution instrument allowing a reproducible testing of APIs, intermediates and final dosage forms. The CE 7smart gives relevant information from pre-formulation and characterization to IPC and QC testing.
Biorelevant methods
Envisioned to mimic in-vivo conditions more accurately than beaker methods, the Flow-Through Cell technique was born to ambitiously push dissolution testing borders. Compatible with biorelevant media from level zero to three, the CE 7smart remains a powerful and flexible tool to change pH on the fly in extended and delayed release dosage form testing. The CE 7smart is also a method of choice for poorly soluble compounds BCS class II and IV.
Volume flexibility
The CE 7smart user can adapt his open and closed system settings to its real solubility and sensitivity method requirements, simply adjusting the required media volume. From 50 to 5000 mL, maintaining the same hydrodynamic conditions in every volume condition, the CE 7smart allows a reproducible positioning of the dosage form into the cell.

Assessing subcutaneous injectable drug release
BioJect™ is a cutting-edge technology developed to evaluate the release of drugs from subcutaneous injectables. It broadens the scope of flow-through cell dissolution testing to include subcutaneous peptide formulations. Leveraging a compendial dissolution framework enhances the capabilities of BioJect™. The 22.6-mm-diameter cell now includes a hydrogel-based (agarose) reservoir, which provides precise and consistent control over pore size and diffusion properties.
