q-doc® – Data Management Software
Single workstations and networked solutions with built-in data integrity.

- 100% scalable 3-layer software architecture with central MS-SQL database
- Powerful framework with device-independent methods that can be used for different instrument types
- Modules extend the basic functionalities with LDAP integration, LIMS export, data trending, and more
- More than 35 drivers for dissolution and physical testing instruments
- Report, check & evaluate data directly in q-doc and sign with electronic signatures
- Easily create complete batch reports and compare batches over time intervals
- Human readable audit trail and product version control for full traceability and compliance
- Fulfills all requirements for implementation of a 21 CFR part 11 compliant system
Highlights

Integrate Instruments.
Standard q-doc® drivers integrate more than 40 instrument types from different brands – controlling test parameters and automatically recording results. Boost lab efficiency and safeguard critical test data with 100 % traceability according to 21 CFR part 11.

Integrate Processes.
Process drivers ensure that proper procedure is followed. Operators are guided through manual testing processes with step-by-step instructions. All user actions and meta data (e.g. date, time, serial numbers) are automatically protocolled and reported.
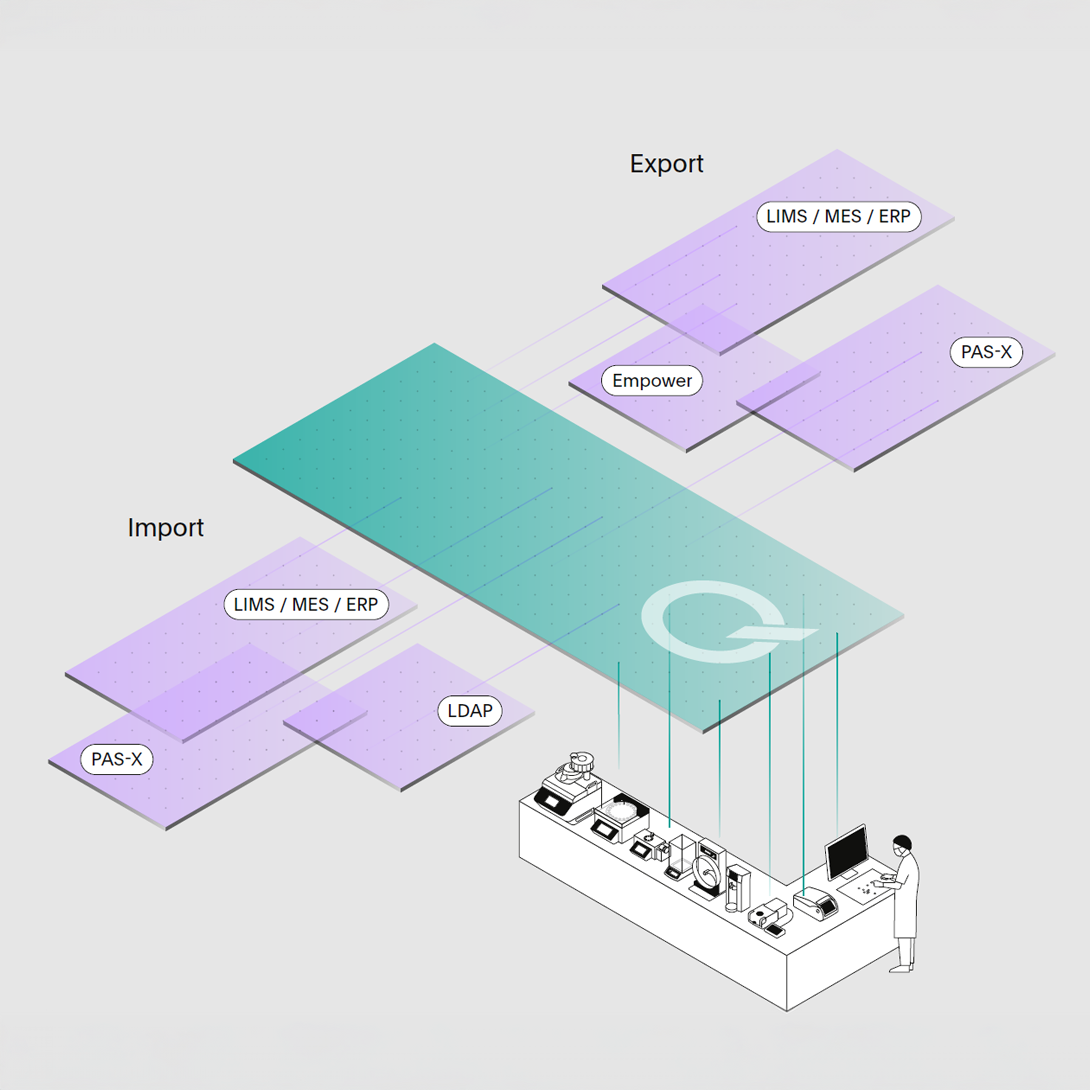
Integrate with your Ecosystem.
Run q-doc® seamlessly integrated with other data management systems for operational efficiency with enhanced traceability across digital platforms.

Empower your AI with q-doc®
Optimize Design of Experiment (DOE) parameters or enable predictive manufacturing. q-doc® delivers structured data ready for your company's Artificial Intelligence (AI) model – turning data into actionable intelligence.

Built-in Data Integrity.
q-doc® automatically tracks who did what, when, and where in an electronic audit trail that can easily be reviewed from any PC within the network. Complete traceability and 100% compliance.

Operational Excellence in Dissolution.
Maximize instrument usage and send test runs to any dissolution tester in your lab. Analyze UV-Vis spectra online – or automatically create sample sets in Empower® CDS to link HPLC results to your dissolution data.

In-Process Control
Streamline in-process control (IPC) for rapid decisions – from raw material testing to powder characterization and testing the physical properties of final dosage forms. q-doc® integrates manual inspections, connects instruments from different brands, and interfaces seamlessly with your MES for fully digital workflows.

Managing large Networks.
q-doc® connects departments to unlock significant cost savings – while simplifying complexity for operators and IT teams. Workgroups, virtualization, mobile access, and instrument pooling deliver clarity, boost flexibility, and strengthen security.

Future-Proof your Installation.
q-doc® software support contracts are all about future-proofing your installation. They combine fast and uncomplicated priority service with free update licences to be ready for tomorrow's challenges without unexpected costs.