News
22 May 2025
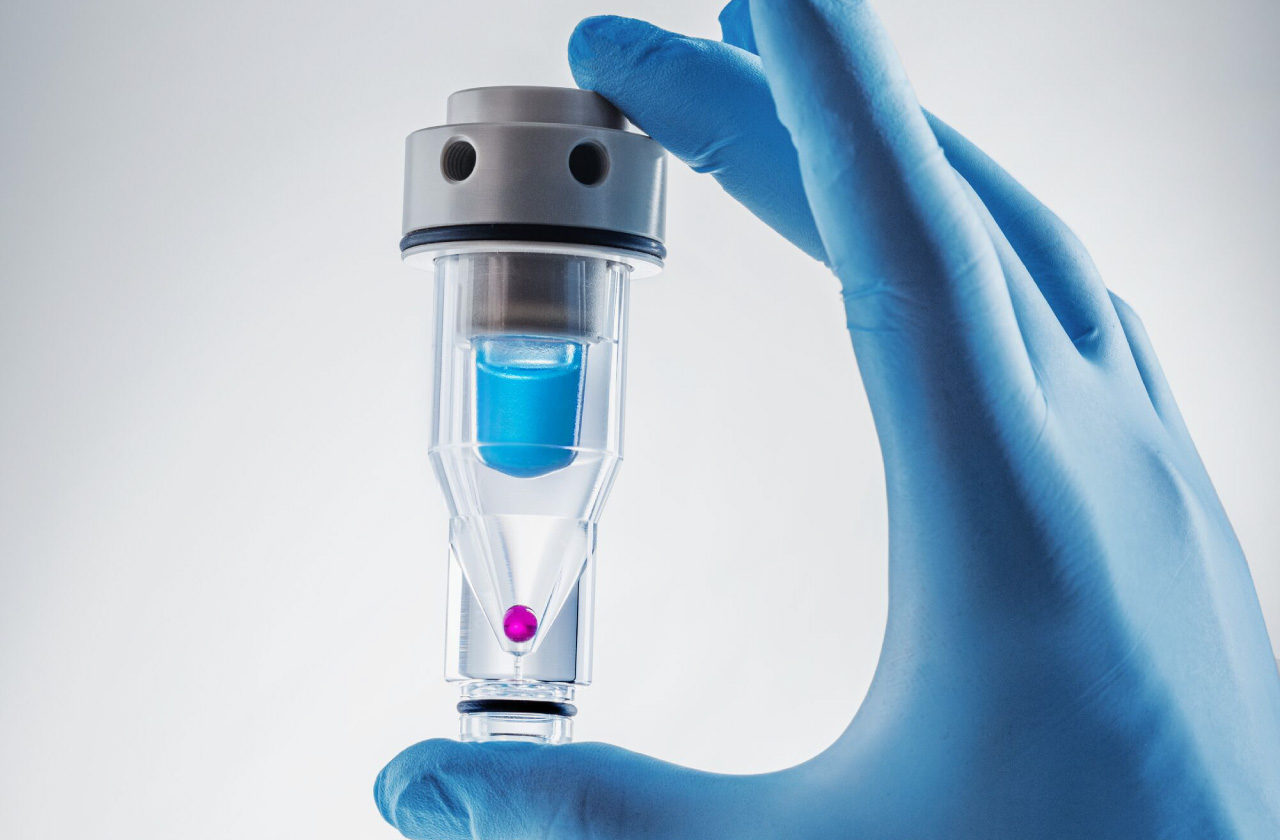
BioJect™ – Innovation for subcutaneous formulation testing
Until now, no in vitro setup could accurately mimic the release of a free drug from a formulation injected subcutaneously in a flexible, reproducible, user-friendly, and QC-ready manner. The new BioJect™ specialized cell for CE 7smart can!
Initially developed by Dr. David Li under the supervision of Pr. Matthias Wacker of the National University of Singapore to study the release and permeation mechanisms of various insulin formulations, BioJect™ is ideally suited for testing a wide variety of subcutaneous injections – from insulin to in-situ forming implants, other peptides and small molecules. Please refer to their scientific publication titled "BioJect: An in vitro platform to explore release dynamics of peptides in subcutaneous drug delivery" in the Journal of Controlled Release Volume 380 to dive deeper into this topic.
BioJect™ (patent pending) for the CE 7smart flow-through cell dissolution tester is a new cell type for drug release testing of subcutaneous injectables. The cell has a small container inside – the so called ‘biomatrix’. The injectable liquid to be tested is filled into this container. Because the walls of the 'biomatrix container' are permeable and similar to subcutaneous skin tissue, the drug is slowly released through the walls of the ‘biomatrix container’ into the medium during the test. For more information on how BioJect™ works, please refer to the recently published application note.