CE 7smart离线收集模式
流池法溶出度测试系统,可自动采集样本。

- 收集 – 将溶出度测试样品收集于试管或小瓶
- 受控 – 可控的流体动力学
- 开环 – 开环配置,具备不受限的漏槽条件,可自动切换不同pH值的溶媒
- 闭环 – 闭环配置,可固定溶媒体积(15-4000毫升),适用于药典规定的低体积溶出度测试
- IVIVC – 采用开环配置简化体内外相关性的研究
- 应用 – 原料药、口服、外用及注射剂型的可重复测试
- 清洁 – 自动化清洁程序
产品亮点

简单高效
离线采集溶出测试样品是实现溶出度测试自动化的最简便方式。使用分裂阀从恒流液中取样。样品由CP泵输送,在流向收集的试管/小瓶过程中,通过流通池头部的圆形滤膜进行过滤。样品存储于C613或C615样品收集器中。此过程中,样品始终以相同的方式处理,因此效率最高。

从原料药到新型制剂
溶出度测试是制剂生产过程中监测参数的重要指标。CE 7smart流池法溶出度测试系统拓展了实验设计范围,是一款能够对原料药、中间体和最终剂型进行可重复测试的溶出仪。CE 7smart 可提供从预配方和表征到过程控制(IPC)和质量控制(QC)测试的相关信息。
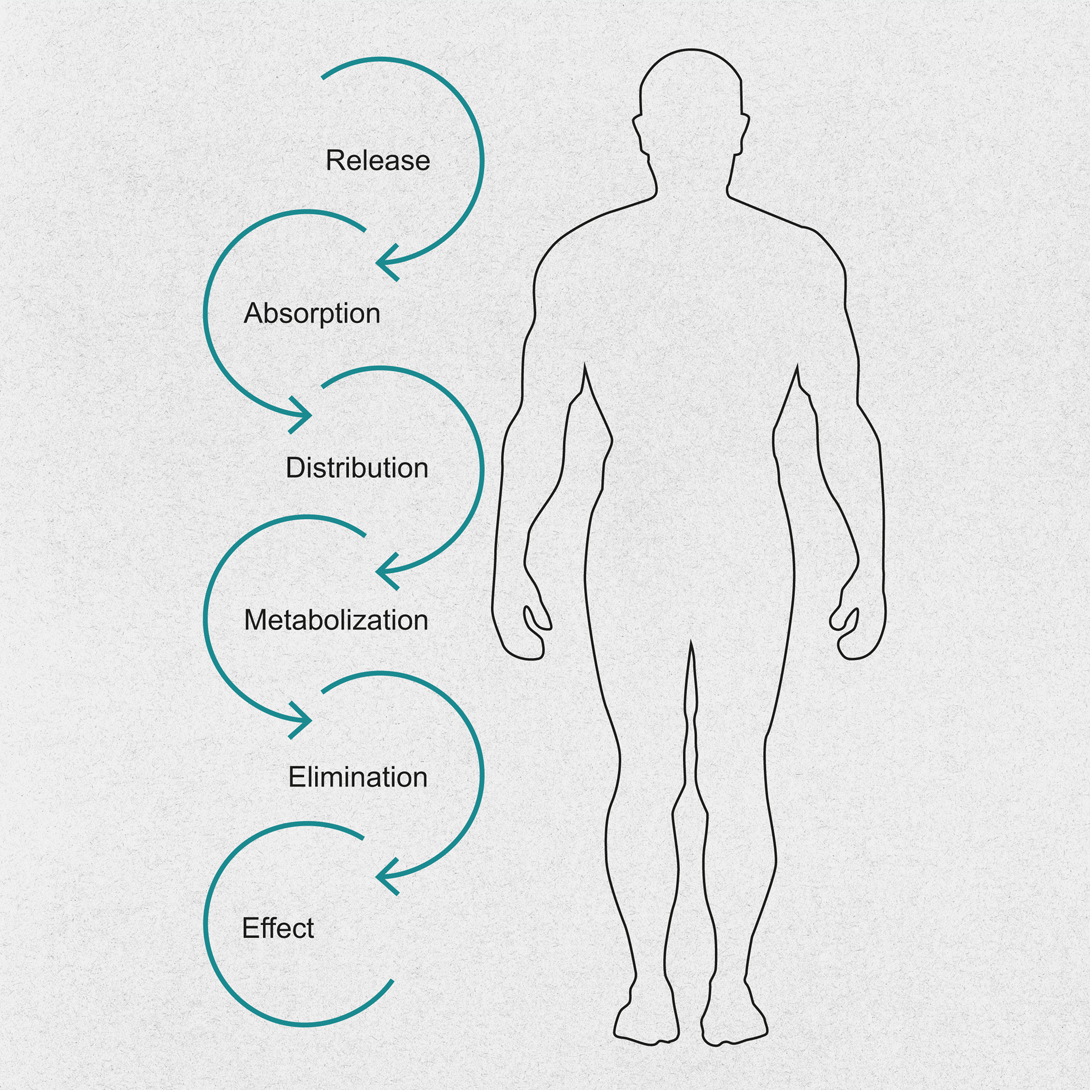
生物等效方法
为了比烧杯法更精确地模拟体内环境,流池法应运而生,以突破溶出度测试的界限。CE 7smart与生物相关介质(零级至三级)兼容,是一款功能强大且灵活的工具,可用在缓控释制剂测试中切换不同pH值的介质。是一款强大且灵活的工具。对于 BCS II 类和 IV 类难溶性化合物,CE 7smart也是首选的方法。
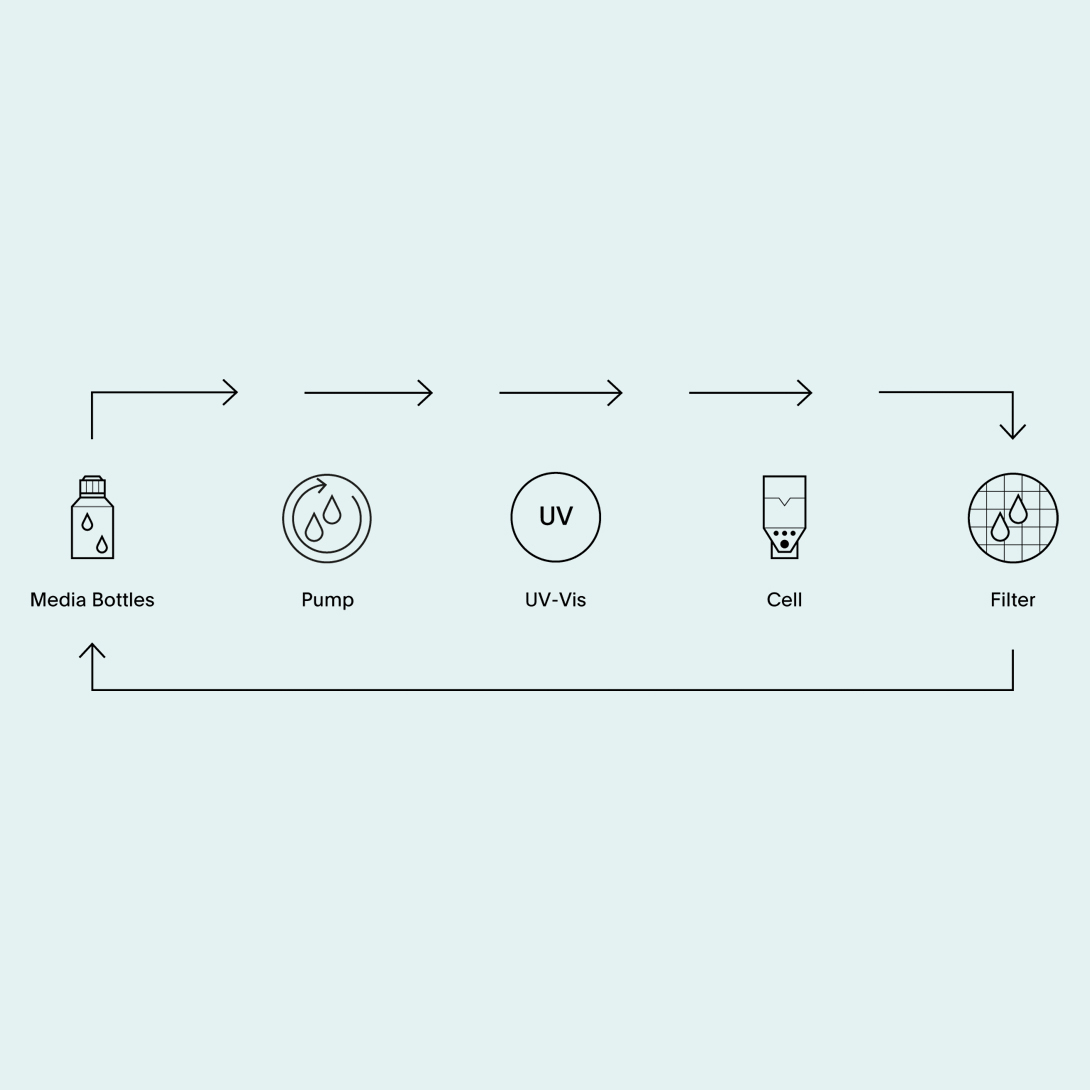
溶媒体积灵活性
CE 7smart的用户可根据实际溶解度和灵敏度方法的要求,选择合适的溶媒体积,调整其开环和闭环系统的设置。CE 7smart的溶媒体积范围为50-5000毫升,在该体积条件下均能保持相同的流体动力学条件,从而实现制剂在流通池中的可重复定位。

皮下注射剂测试
BioJect™是一款专为评估皮下注射剂药物释放而设计的专用流通池适配器。它将流池法溶出度测试的应用扩展至皮下肽类制剂。BioJect™基于药典溶出度测试装置构建,进一步拓展了溶出度测试的功能。在内径为22.6mm的流通池中加入水凝胶基质储液池(琼脂糖),可精确且可重复地控制其孔径大小与扩散行为。
